Pooled protein tagging and optical in-situ sequencing for characterizing proteome dynamics at scale.
System-level understanding of cellular organization requires methods for direct visualization of proteins at scale. We have developed methods for pooled protein tagging to generate complex cell libraries such that in each cell a different, single, protein is endogenously tagged (check out the website background image!!). We use high-throughput imaging, optical barcode sequencing and deep learning based image analysis to uncover the identity of the tagged protein and cluster localization patterns of hundreds of proteins. This approach provides us with a birds-eye-view of proteome organization which we can use to chart changes in cellular organization in response to environmental or genetic perturbations in an unprecedented scale.
Regulation and function of the Parkinson’s Disease protein alpha-synuclein
Alpha-synuclein is a major component of Lewy-pathology (LP), which is the hallmark of Parkinson’s Disease (PD) and related neurodegenerative diseases (ND), with alpha-synuclein protein levels alone being strongly associated with PD risk. Despite the central role that alpha-synuclein plays in the pathogenesis of PD and other NDs, the function and regulation of the protein remains poorly understood. In a study soon to be published, we engineered cell line and iPSC derived neuronal models to efficiently identify regulators of alpha-synuclein protein levels at an unbiased manner. We are continuing these efforts and also using additional functional genomics and biochemical approaches to better understand how the protein is regulated and gain insight into its elusive molecular function.
The formation and downstream consequences of protein aggregation
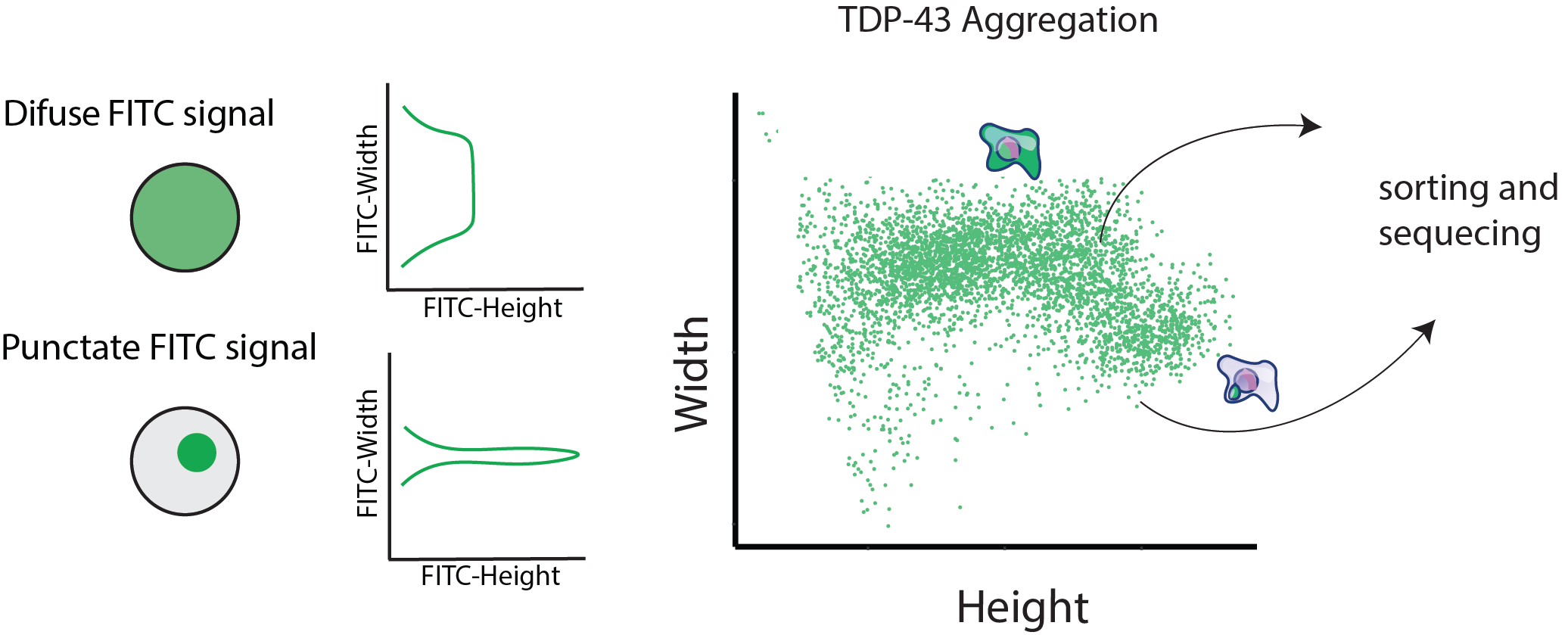
The presence of large protein inclusions is a major pathological hallmark of most neurodegenerative diseases. Using FACS based and optical pooled CRISPR screens we aim to better understand the cellular factors that sensitize or protect cells from forming large protein inclusions. In addition, we are very interested in understanding the downstream consequence of protein aggregation and how it contributs to neuronal toxicity and death.
Pooled optical screening in neuronal models of neurodegenerative diseases
Neuronal phenotypes associated with neurodegenerative diseases, especially in early stages, can be very subtle, requiring high resolution fluorescence microscopy and image analysis to identify and screen for modifiers. To scale screening in iPSC derived neuronal models we are developing the required tools for pooled optical screening in cells with complex morphologies and use them to characterize the effect of genetic perturbations on basic neuronal cell biology processes and disease associated phenotypes.
Direct perturbation and recruitment of endogenous proteins
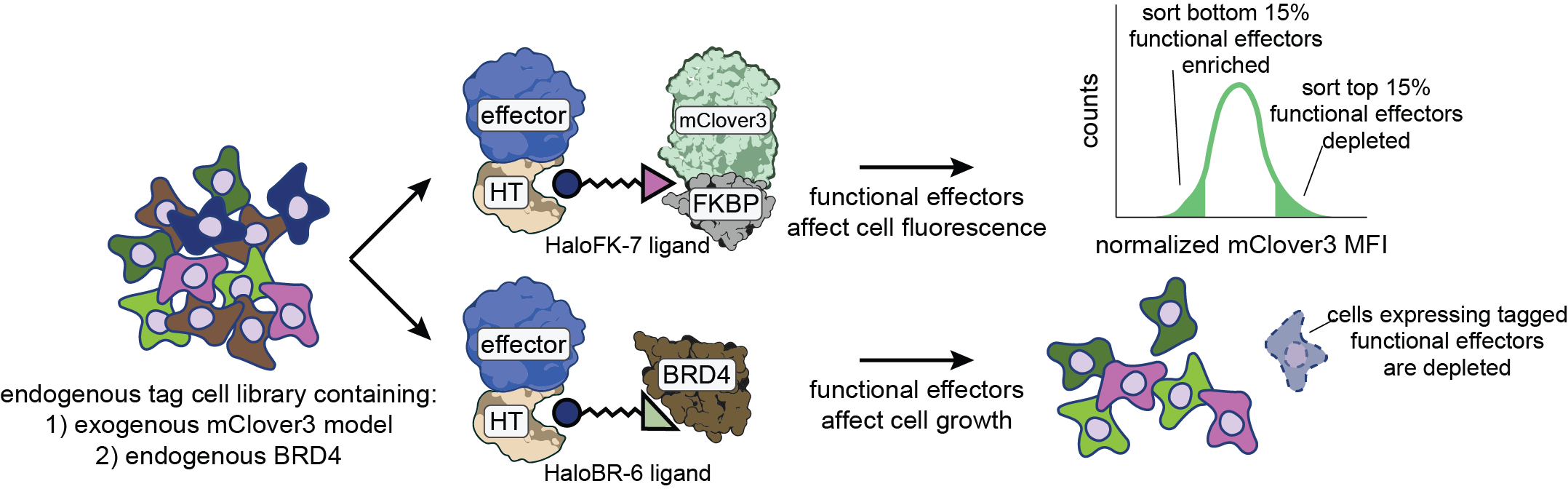
A wide range of tools are currently available to directly target the DNA (using CRISPR) or RNA (using shRNA and CRISPR) yet directly targeting proteins at scale remains a major challenge. Using our pooled tagging technologies we have developed SPOTLITES: POoled Targeting with a LIgandable Tag at Endogenous Sites. Using this approach we can fuse endogenous proteins to a protein tag that can be bound with a heterobifunctional molecule. Such molecules bind the target protein on one side to induce different perturbations by having different effector molecules on the other. These can range from small molecule fluorophores, destabilizers and a range of other effects by recruiting other endogenous protein factors. We are also using similar approaches to recruit a wide range of effector proteins to a single target protein for the development of novel proximity based therapeutics.
Generating a holistic view of RNA condensation and membraneless organelles in cells and neurons
Cellular stress initiates fast and transient changes in the translation landscape by sequestering mRNAs into stress granules in which translation is inhibited while only few transcript preferentially form active polysomes. Dysfunction of this process has been strongly linked to several neurodegenerative diseases, most prominently ALS and FTD. Using our pooled gene tagging and in-situ sequencing approaches we are able to map changes in localization patterns of hundreds of RNA binding proteins to identify novel stress associated granules and map the cross dependency of multiple granules by tagging more than one protein in each cell.
More to be added!!